ISO 13485:2016 CQI-IRCA Lead Auditor Course Aim
This course equips participants with the foundational information and assessment techniques that will benefit organizations. The requirements of PR 369 are met by this course. Individuals seeking Third Party Assessments are satisfied with the formal training requirement after successfully completing the course.
How will your benefits?
Knowledge of the following quality management principles and concepts:
- The PDCA cycle (Plan, Do, Check, Act).
- The connection between the relevant international regulatory standards for medical devices and ISO 13485.
- Terms and definitions related to quality management are found in ISO 13485 and ISO 9000.
- A practical understanding of medical device rules, regulatory auditing standards, and their connections to ISO 13485 that are applicable to the nations the course is planned to cover.
- A CQI and IRCA course related to specific regulatory authority standards, which will be made accessible based on demand (for example, MD-QMS Comprehensive EU Medical Device Regulation 2017/745 (EU MDR) Practitioner - PT219), can be used to gain this expertise.
- A practical understanding of risk-management guidelines relevant to medical device design.
 Course Content
Course Content
- Auditing Process Approach Including the PDCA Cycle ISO 13485:2016 Standard Requirements
- Documentation of Medical Device Quality Management Systems is being audited.
- ISO 19011 standard standards auditing
- Auditor's Auditing Roles and Responsibilities
- Audit Planning- Audit Strategy
- Checklists and Non-Compliance Reports
- Reporting on Audits
- Meeting of the Final Team/Follow-up
- Audit/Verification Records
- Exercises, Case Studies, and so forth.
- Auditor Registration and Accreditation/Certification
PDCA Cycle
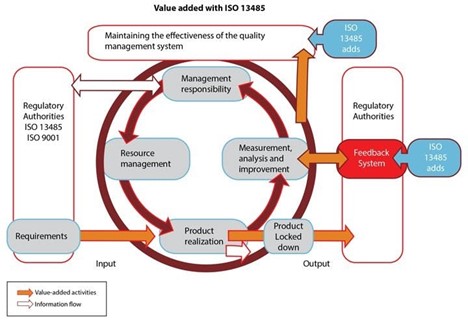
.
Who Should Attend This Course?
- A Lead Assessor or Third-Party Assessor of ISO 13485:2016
- accountable for conducting supplier/subcontractor audits in accordance with ISO 13485:2016
- Implementing internal audits and audit programs in accordance with ISO 13485:2016 is your responsibility.
- accountable for putting the ISO 13485 family of standards into practice
Course Outline
Course starts at 0830 Hrs. and ends approximately at 1800 Hrs. Maximum attendance 12 delegates per tutor and maximum 20 delegates with 2 tutors.
Lunch Break :45 min.
Coffee Breaks: Mid-morning & Mid-afternoon (Max. 10 min. each)
Pre-course information is sent to delegates approximately a week prior to the beginning of the course
Course Facilitators
All course facilitators are highly qualified and experienced both in training and assessment of Medical Device Quality Management System. We strongly believe that the experience in Medical Device Quality Management System Assessment and Training skills is a right combination to deliver a practical training course. They are selected on their ability to make BSCIC courses both practical and enjoyable.
Our training sessions are available in both virtual and on-site formats to meet your unique preferences.
Pre-Course Knowledge (Recommended)
- Basic understanding of management system principles and the Plan-Do-Check-Act (PDCA) cycle
- Familiarity with the structure and key concepts of the relevant ISO standard
- Awareness of process-based approaches and management system terminology
- Prior exposure to auditing activities (e.g., internal audits) is preferable
- Graduation and 1–2 years of work experience in a relevant field (preferred but not mandatory)
- Completion of a Foundation Course is recommended (though not mandatory) and may also be achieved through self-study
To Enquire for This Course Please Click Here
